Entry to essentially the most generally used technique of abortion in the USA plunged into uncertainty on Friday following conflicting court docket rulings over the legality of the abortion medicine mifepristone that has been extensively obtainable for greater than 20 years.
For now, the drug that the Meals and Drug Administration (FDA) accredited in 2000 stays no less than instantly obtainable within the wake of the separate rulings that have been issued simply minutes aside by federal judges in Texas and Washington state.
U.S. District Choose Matthew Kacsmaryk, a Trump appointee, ordered a maintain on federal approval of mifepristone in a call that overruled a long time of scientific approval. However that call was rapidly adopted by U.S. District Choose Thomas O. Rice, an Obama appointee, primarily ordering the alternative and directing U.S. authorities to not make any modifications that will limit entry to the drug.
The extraordinary timing of the competing orders revealed the excessive stakes that encompass the drug a yr after the U.S. Supreme Court docket overturned Roe v. Wade and curtailed entry to abortion throughout the nation.
Accelerated path to Supreme Court docket possible
The Justice Division swiftly gave discover it will enchantment the Texas ruling and stated it was reviewing the choice from Washington state.
The whiplash of the conflicting selections is more likely to put the problem on an accelerated path to the Supreme Court docket.
“FDA is beneath one order that claims you are able to do nothing and one other that claims in seven days I’ll require you to vacate the approval of mifepristone,” stated Glenn Cohen of Harvard Legislation College.
A conservative Texas choose is now contemplating arguments in a case that might ban the generally used abortion invoice mifepristone in the USA. Final November, a coalition of anti-abortion teams filed a lawsuit to reverse federal approval of the tablet.
Abortion suppliers slammed the Texas ruling, together with Entire Lady’s Well being, which operates six clinics in 5 states and stated it will proceed to dispense mifepristone in individual and by mail over the following week because it critiques the rulings.
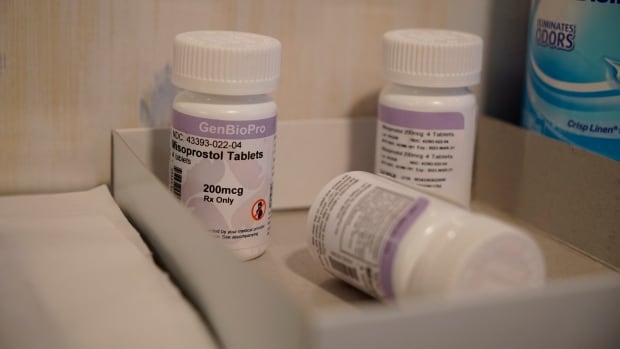
The abortion drug has been extensively used within the U.S. since 2000, and there’s primarily no precedent for a lone choose overruling the medical selections of the FDA. Mifepristone is one among two medication used for medicine abortion in the USA, together with misoprostol, which can be used to deal with different medical circumstances.
Kacsmaryk signed an injunction directing the FDA to remain mifepristone’s approval whereas a lawsuit difficult the protection and approval of the drug continues. His 67-page order gave the federal government seven days to enchantment.
“Immediately’s choice overturns the FDA’s knowledgeable judgment, rendered over 20 years in the past, that mifepristone is secure and efficient,” U.S. Legal professional Common Merrick Garland stated. “The division will proceed to defend the FDA’s choice.”
Different drug obtainable
Medical doctors and clinics that prescribe the two-drug mixture have stated that if mifepristone have been pulled from the market, they’d change to utilizing solely the second drug, misoprostol. That single-drug method has a barely decrease charge of effectiveness in ending pregnancies, however it’s extensively utilized in nations the place mifepristone is against the law or unavailable.
The lawsuit within the Texas case was filed by the Alliance Defending Freedom, which was additionally concerned within the Mississippi case that led to Roe v. Wade being overturned. On the core of the lawsuit is the allegation that the FDA’s preliminary approval of mifepristone was flawed as a result of it didn’t adequately assessment its security dangers.
Courts have lengthy deferred to the FDA on problems with drug security and effectiveness. However the company’s authority faces new challenges in a post-Roe authorized setting during which abortions are banned or unavailable in 14 states, whereas 16 states have legal guidelines particularly concentrating on abortion medicines.

Because the Texas lawsuit was filed in November, authorized consultants have warned of questionable arguments and factual inaccuracies within the Christian group’s submitting. Kacsmaryk primarily agreed with the plaintiffs on all of their main factors, together with that the FDA did not adequately assessment mifepristone’s security.
“The court docket doesn’t second-guess FDA’s decision-making calmly,” Kacsmaryk wrote. “However right here, FDA acquiesced on its professional security issues — in violation of its statutory obligation — based mostly on plainly unsound reasoning and research that didn’t assist its conclusions.”
Mifepristone has been utilized by thousands and thousands of ladies over the previous 23 years, and problems from mifepristone happen at a decrease charge than that seen with knowledge tooth removing, colonoscopies and different routine medical procedures, medical teams have lately famous.
Elsewhere, Kacsmaryk sided with plaintiffs in stating that the FDA overstepped its authority in approving mifepristone, partly, by utilizing a specialised assessment course of reserved for medication to deal with “critical or life-threatening diseases.” The choose brushed apart FDA arguments that its personal rules clarify that being pregnant is a medical situation that may typically be critical and life-threatening, as an alternative calling it a “pure course of important to perpetuating human life.”