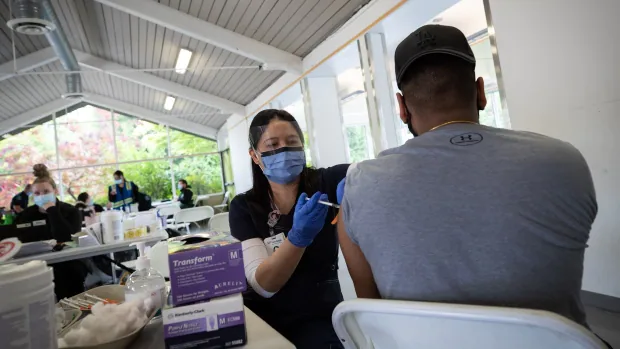
Advisers to the U.S. Meals and Drug Administration on Tuesday really helpful a change within the design of COVID-19 booster photographs this fall with a view to fight extra not too long ago circulating variants of the coronavirus.
The FDA’s Vaccines and Associated Organic Merchandise Advisory Committee voted 19-2 that the following wave of COVID booster photographs ought to embrace a part that targets the Omicron variant of the coronavirus.
The FDA must resolve the precise recipe, however anticipate a mix shot that provides safety towards Omicron or a few of its newer relations to the unique vaccine. The company plans to resolve by early July on what the design of the boosters must be.
It is also not but clear who could be provided a tweaked booster — they could solely be urged for older adults or these at excessive threat from the virus.
FDA scientists on the assembly recommended they most well-liked vaccines that may goal the BA.4 and BA.5 Omicron subvariants, which are at present dominant, somewhat than the BA.1 Omicron variant that led to an enormous surge in infections final winter.
The fast-spreading BA.4 and BA.5 sub-variants of Omicron are estimated to make up a mixed 52 per cent of the coronavirus instances in the USA as of June 25, the U.S. Facilities for Illness Management and Prevention (CDC) stated on Tuesday. The 2 sublineages accounted for greater than one-third of U.S. instances for the week of June 18.
Dr. Isaac Bogoch, an infectious ailments specialist at Toronto Common Hospital, advised CBC Information in an interview broadcast Tuesday that BA.5 represents “in all probability about 20 per cent of all COVID proper now in Canada.” Well being Canada monitoring as of June 24 had tracked BA.5 in 15 per cent of all COVID-19 infections within the nation, with eight per cent of instances attribute of BA.4.
‘Higher match’ to current strains wanted
Dr. Peter Marks, director of the FDA’s Middle for Biologics Analysis and Analysis, stated the regulator would hope to launch a booster marketing campaign with a retooled vaccine by October.
“The higher the match of the vaccines to the circulating pressure we imagine might correspond to improved vaccine effectiveness, and doubtlessly to a greater sturdiness of safety,” Marks advised the assembly of out of doors skilled advisers to the company.
Dr. Kanta Subbarao, representing a World Well being Group advisory committee that additionally thought of the difficulty, stated she most well-liked BA.1-based vaccines, suggesting they may generate a broader immune response as a result of that variant is extra distinct from the unique virus than its successor subvariants.
“Our objective right here is to attain broader immunity towards circulating and rising variants,” Subbarao stated, noting that making an attempt to match what variant may be circulating within the fall is troublesome due to uncertainty concerning the trajectory of the evolution of the virus.
Scientists in Canada and world wide are attempting to resolve a COVID-19 thriller — why have some folks by no means examined constructive, regardless of being uncovered? And will they be genetically immune to the virus?
Encouraging uptake to a public fatigued by over two years of the pandemic shall be a problem. Solely half of vaccinated People have acquired a primary booster. Just one-quarter of these eligible for an extra booster have taken it up.
In Canada, Well being Canada monitoring signifies that almost 56 per cent of these 12 and older are thought of absolutely vaccinated, with at the very least one further dose.
Eligibility for an extra dose past that has diverse by province. Nationally, 20 per cent of these 60 to 69 are thought of absolutely vaccinated with two further doses, with that charge virtually 42 per cent for these 70 to 79, and practically 50 per cent for these 80 and over.
Well being Canada’s vaccine advisory committee subsequent meets on July 4-5.
Vaccine makers current findings on new formulation
Pfizer, Moderna and Novavax introduced knowledge on the U.S. assembly. All three firms have been testing variations of their vaccines up to date to fight the BA.1 Omicron variant.
Moderna stated it might be prepared with a “couple of hundred million” of bivalent, or double-targeted, vaccines designed to fight BA.1 by September. It could be late October or early November if it must design a vaccine concentrating on the newer subvariants, the corporate stated.
Pfizer stated that it and associate BioNTech already has a major quantity of BA.1 vaccine prepared and is making ready to supply a considerable amount of vaccine concentrating on BA.4 and BA.5. It stated both might be prepared for an early October rollout.
Each Moderna and Pfizer have stated that their respective BA.1-inclusive vaccines generated a greater immune response towards Omicron than their present photographs, which have been designed for the unique virus that emerged from China.
They’ve stated their new vaccines additionally seem to work towards BA.4 and BA.5, however that safety just isn’t as sturdy as towards BA.1.
Novavax, in the meantime, is awaiting FDA authorization of a extra conventional form of COVID-19 vaccine, with protein-based photographs. Novavax argued Tuesday {that a} booster of its common vaccine guarantees a very good immune response towards the brand new Omicron variants with out a recipe change.
The Novavax vaccine, often known as Nuvaxovid, was approved to be used in Canadians 18 and over by Well being Canada in February.